The Goudy Regenerative Research Lab is focused on understanding the basic biologic mechanisms that control facial bone and soft tissue regeneration. Dr. Goudy’s clinical job involves repair of craniofacial malformations including cleft lip, cleft palate and Pierre Robin sequence, and he also participates in head and neck tumor resection and reconstruction. In both scenarios, children need cutting edge therapies to improve wound healing, reduce operative complications, and develop novel therapies to restore function and reduce morbidity. Our lab is focused on modeling bone and soft tissue loss in the craniofacial skeleton and we have developed models that allow us to study this in depth. Using these models, the Goudy lab collaborates with multiple labs at both Emory and Georgia Tech to further develop regenerative approaches to craniofacial bone formation and soft tissue repair following injury. Specifically, these regenerative therapies include: delivery of Jagged1 in PEG-MAL hydrogels to repair bone; delivery of FTY720-loaded biomaterial scaffolds to repair oral cavity mucosa; delivery of commensal bacteria using hydrogels to enhance oral wound healing. Our goal is to push the boundaries of what is currently known about facial bone and tissue repair, and ultimately use models to provide translational, clinical relevance, all while having fun!
Current Lab Collaborations:
Dr. Nick Willett • Dr. Dennis Liotta • Dr. Michael Davis
Dr. Hicham Drissi • Dr. Rabindra Tirouvanziam • Dr. Shelly Abramowicz
Dr. Edward Botchwey • Dr. Scott Hollister • Dr. Vahid Serpooshan
Dr. Luke Brewster • Dr. Jones Rheinallt
Steven Goudy, M.D., MBA is the Director of Pediatric Otolaryngology at Emory University, where he is focused on craniofacial bone development and regeneration. His lab is currently developing regenerative approaches to pediatric craniofacial bone loss using animal models. Using in vitro and in vivo approaches, Dr. Goudy is developing innovative approaches for drug and cell delivery, leveraging their collaborations with the Georgia Institute of Technology. Aside from spending time in lab and clinic, he is an active runner and was one of the Olympics torch-bearers at the 1996 Centennial Olympic Games.
Hope Robinson, Ph.D. comes to us from the cancer side of research, having earned her PhD in Cancer Biology and completing a post-doctoral fellowship in Cancer Immunology here at Emory. She is excited to work on encouraging cells to grow and divide rather than figuring out better ways to eliminate them. Hope and her family have lived in Georgia for over 25 years and love Atlanta. When she is not doing research, Hope enjoys spending time with her husband, their adult children, and the various animals that have found their way to the Robinson home.
Afra Toma, M.S. is currently a Ph.D. Candidate in the Joint Biomedical Engineering at the Georgia Institute of Technology and Emory University. She graduated with her BS and MS degrees in Biomedical Engineering from Florida International University and University of Florida, respectively. As Toma is co-advised by Dr. Steven Goudy and Dr. Nick Willett, her project focuses on developing biomaterials-based immuno-regenerative therapies for orofacial clefts in pediatric patients and enhancing oral wound healing via local delivery of immunomodulatory drug. As she progresses through her PhD, Toma aspires to master trades that will allow her to empower the scientific community as a researcher, leader, and role model. Apart from spending time in lab, Toma enjoys photography, cooking, and painting.
Keerthi Priya Chinniampalayam Sekar, Ph.D. is currently a postdoctoral fellow. I relocated here due to my spouse pursuing a postdoctoral training. I earned my PhD, M.S, and B.S degrees from the University of Madras. My doctoral and M.S projects primarily focused on stress and behavior in various models, such as sleep deprivation and adolescent stress. For my postdoctoral work, I am dedicated to studying the role of the oral microbiome in wound healing, particularly in oronasal fistula models. Outside of academics, I enjoy cooking, gardening, traveling, trying different cuisines, and playing chess.
Sundus Kaimari, B.S. is currently an M.S. student in Biomedical Engineering at Georgia Tech. She earned her B.S. in Biological Engineering in 2021 from the University of Georgia. Her research interest is the use of biomaterial in the context of tissue regeneration. Her project focuses on the development of a high throughput assay that screens for activators of p70 S6K to harness them in craniofacial bone regeneration. In the future, Sundus hopes to expand her knowledge in regenerative therapies and apply that in providing therapies for pediatric patients. Outside of lab, Sundus enjoys hiking, playing badminton, reading, and spending time with friends.
Tim Cha, B.S. earned his B.S. in Mathematical Biology and Biopsychology, Cognition, and Neuroscience (BCN) at the University of Michigan, 2020. While at Michigan, he worked as a lab manager for a neuroscience lab studying sensory processing, motivation, and homeostatic behaviors. He moved to Emory with his partner in 2023 and was fortunate enough to join the Goudy Lab! Tim’s research interests include animal models of disease, histology, and the ethical care and use of animals in research. In the future, Tim will be applying to veterinary school. Besides time spent in lab, he loves to hike and camp with his two Shiba Inus, bake, try new restaurants, and run outside (not on a treadmill).
Former Members:
Yejin Katy Suh, B.S. recently completed her B.S. in Biology at Emory University, 2023. Her current project focused on elucidating the pathways contributing to the effects of Bone Morphogenic Protein-2 (BMP2) on human pediatric bone cells. In the near future, Katy will be applying to medical school. Outside of the lab, Katy enjoys working as an EMT for the Emory community, running, and visiting new cafes and bakeries in Georgia!
Andre Burnham, B.A. earned his undergraduate degree in Biochemistry and Molecular Biology from Hamilton College and is now finishing his M.D. at Emory University School of Medicine. Prior to attending medical school, he worked as a Research Specialist and undergraduate biology lab instructor at Emory University. His laboratory research focuses on studying immunomodulatory properties of small molecules and cell therapies. His clinical research interests include head and neck cancer, postoperative wound healing, and quality of life outcomes in surgical patients. Outside of the lab, he enjoys playing piano, golfing, and beekeeping.
Archana Kamalakar, Ph.D., M.S. was an Associate Scientist in our lab. She hails from India where she completed her Masters in Microbiology and a P.G. Diploma in Clinical Research. Archana discovered her propensity for Science at a very early age and she presented herself as a scientist at her 6th grade career exhibition. Thereafter, she completed her doctoral thesis project titled "Novel Regulators of Osteoclastogenesis: Interleukin-8 (IL-8) and Parathyroid hormone-related protein (12-48), (PTHrP(12-48))” at the University of Arkansas for Medical Sciences. Archana completed her Post-doctoral training under the mentorship of Dr. Goudy. Archana aspires to continue academic research and teaching in order to inculcate the love for Sciences in generations to come. Apart from Science, Archana also has a passion for Sailing. In 2006, she won the gold at the National Sailing Regatta (India) held by the Laser Class Association of India (LCAI).
Heath Bradley, M.S. earned a B.S. in Molecular Biology from Grove City College in 1997 and earned a M.S. in Biotechnology from Johns Hopkins University in 2006. Heath has over 20 years of experience in both research and clinical labs. In the fall of 2021 Heath joined the Goudy lab at Emory as to study the role that the oral and nasal microbiomes play on wound healing of orofacial clefts. Heath hopes to further the lab's study of wound healing to help those in need find the help to have a better life. Outside of work, Heath enjoys outings with friends and family to see the Atlanta United and enjoys camping and other outdoor activities with his family.
Monica Behara, B.S. was an M.S. student in Biomedical Engineering at Georgia Tech. She graduated in 2021, with a B.S. in Biomedical Engineering and a minor in Materials Science Engineering from Georgia Institute of Technology. She is very passionate about the field of immunomodulation and is hoping to expand her knowledge of wound healing in the context of biomaterials implantation. In the future, Monica aspires to work with nanoscale biomaterials, and hopes to eventually apply this technology to immuno-regenerative therapies. In her free time, Monica is a classically trained violinist and pianist, is a member of several orchestras on/off campus, and enjoys singing in a variety of languages.
Nithin Ravikumar, M.S. was an M.S. student in Biomedical Engineering at Georgia Tech with a B.S. in Chemical and Biomolecular Engineering also from the school. In the Goudy Lab, he is currently developing a PEG hydrogel-based strategy for the delivery of Jagged1 to improve bone regeneration of craniofacial injuries. Nithin hopes to strengthen his skills as a researcher and engineer to contribute to the scientific community and play role in improving human health. His other interests outside of science include reading and traveling.
Angelica Amanso, Ph.D., was an academic research assistant scientist. She is from Brazil and obtained her degree in Pharmacy-Biochemistry at Sao Paulo University. Deep understanding of human biology was one of her major interests, so she pursued career as a scientist at the Heart Institute-Sao Paulo University, where she completed her Ph.D. in Cardiovascular basic science. Since 2009, she has been working at Emory University, developing projects and collaborating with several labs. In her spare time, she enjoys nature trails with her four-paw kids.
Samir Ballestas Naissir, M.D., was a medical doctor coming from Universidad del Norte at Barranquilla, Colombia. He discovered his passion for medicine, at a very young age and pursued medical training, specifically in Otolaryngology. Interested in basic science research, he is very enthusiastic about surgical approaches like, voice surgery, endoscopic cranial base tumor resections and cleft palate charity surgeries for underserved communities. One of the purposes of the work he is doing here can also have an impact in vulnerable populations in his country and Latin America. Soccer and reading are among his interests outside medicine and science.
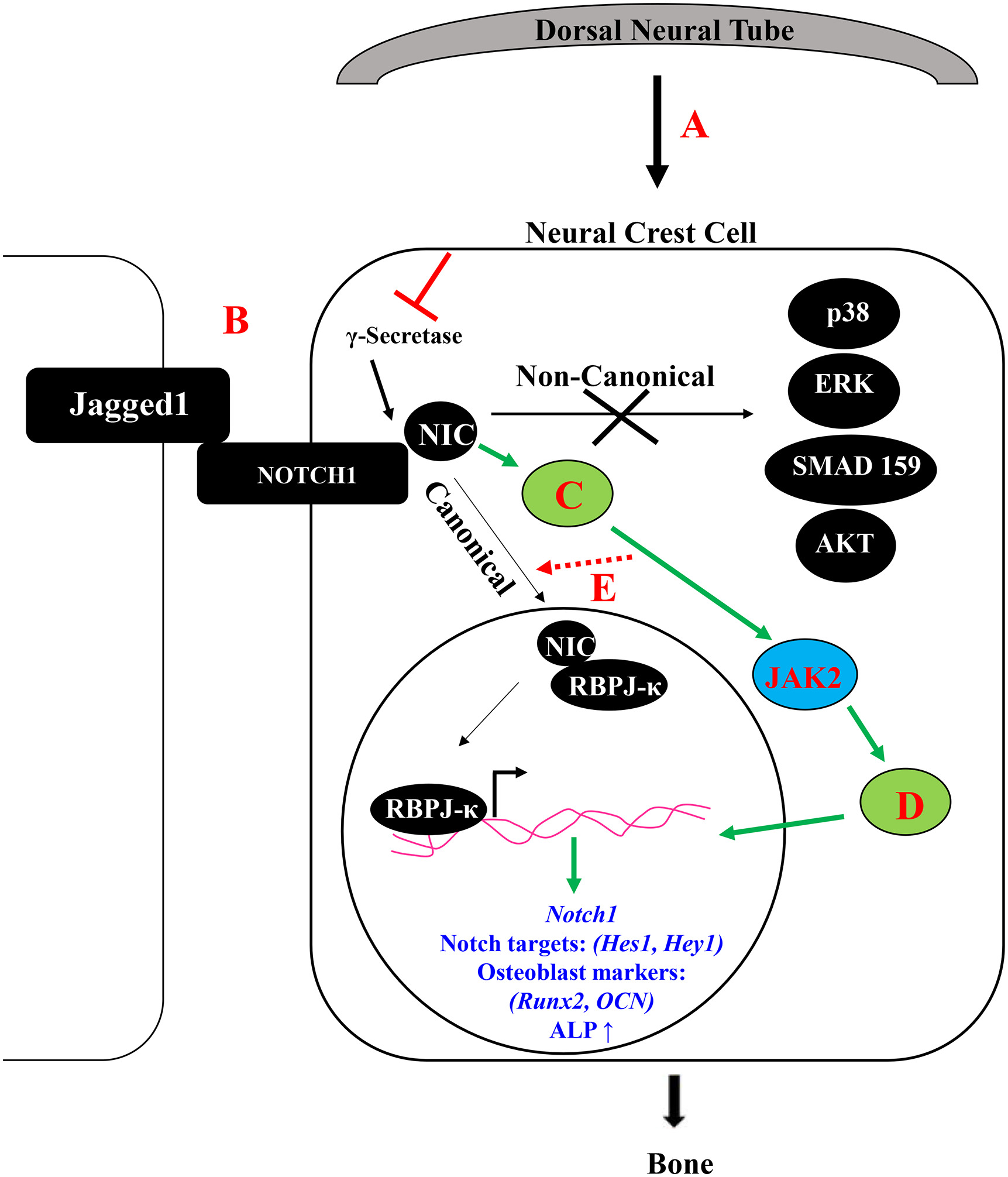
Craniofacial bone loss in children is a challenging clinical problem for which no regenerativestrategies are available. This type of bone loss is primarily treated with autologous bone grafting. Congenital absence of bone in the maxillary area is a defining facial characteristic of Alagille syndrome patients and may require bone grafting to restore maxillary alignment. Alagille syndrome is an autosomal dominantly inherited disorder that occurs primarily due to mutations in JAGGED1 and is associated with cardiac, biliary and bone phenotypes. Children with Alagille syndrome not only have maxillary bone hypoplasia, they also suffer from frequent bony fractures. Adults with Alagille syndrome have reduced bone mineral density and genome wide association studies revealed Single Nucleotide Polymorphisms in JAGGED1 were associated with reduced bone mineral density in otherwise unaffected adults. Together these data highlight the requirement of Jagged1 signaling during bone formation and that its absence causes multiple bony phenotypes, most noticeably maxillary hypoplasia. Jagged1 regulates multiple cell functions including cell survival, cell migration, and cell fate determination. Our initial work has established the requirement of Jagged1 during cranial neural crest (CNC) bone formation through the generation of a mouse model of Alagille syndrome with maxillary bone loss.
Publications:
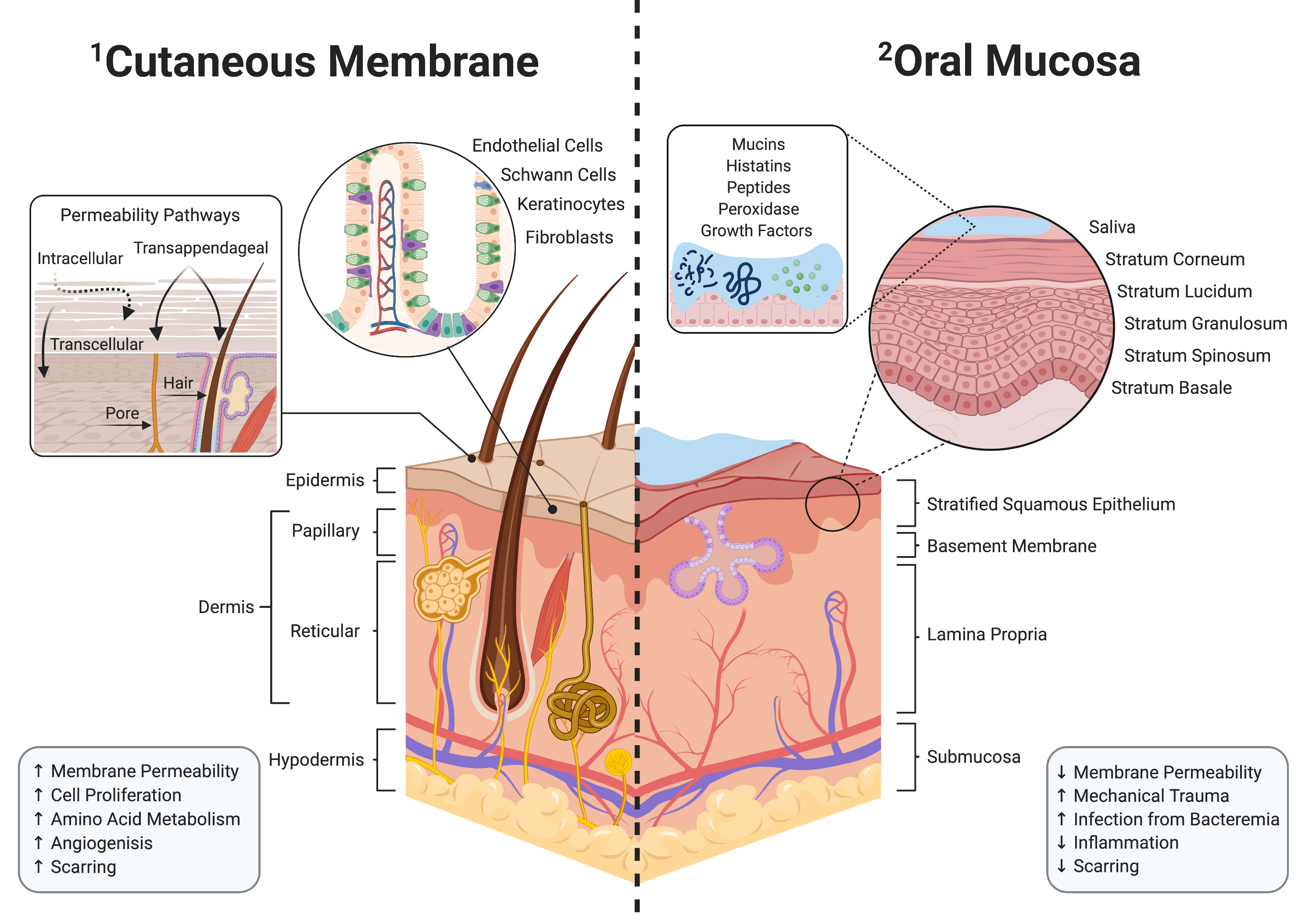
Orofacial clefts are the most prevalent congenital defect and require palate surgery to allow proper feeding and maxillary growth. Due to adverse healing, 60% of these surgeries fail, leading to oronasal fistula (ONF). The ONF affects the child’s ability to eat, talk, and thus, the overall quality of life. Current clinical care to repair ONF uses human donor tissue but carries risk of infection and allograft rejection. As the oral microbiome is bacteria laden, proper wound healing is difficult without immunomodulatory intervention. Along with our collaborators, Drs. Nick Willett, Edward Botchwey, and Dennis Liotta we recently showed that locally delivering immunomodulatory drugs using scaffolds can promote a pro-regenerative oral environment and reduce off-target side effects. We hypothesize that delivering FTY720-loaded polymer scaffolds will enhance oral wound healing and reduce the occurrence of ONF. Integrating principles from both cellular and tissue engineering, we aim to characterize the mechanism by which localized delivery of FTY720 promotes regenerative environment through recruitment of pro-regenerative immune cells. These findings are of extreme importance as harnessing the effects of immunomodulation for oral wound healing provides greater implication for more personalized and efficacious treatment options for pediatric patients.
Publications:
Wound healing has been studied extensively on the skin and particularly following intestinal injury. Intestinal wound healing efficiency is highly sensitive to environmental factors, especially the microbiome, where specific microbial community structures or supplementation with probiotic bacteria enhances the wound healing process. However, little is known about how the oral microbiome affects ONF wound healing. We directly address this gap in knowledge and show preliminary data that creating an ONF in a murine model results in marked changes in the microbiome composition with the complete disappearance of certain microbes and blooms of other bacterial taxa.Thus, our overall hypothesis is that the oral commensal microbiome exerts positive modulatory influences on oral wound healing and may limit ONF formation. Discovering specific bacterial taxa within the oral cavity or wound tissue that positively influence healing will be essential information for the characterization of an eubiotic oral microbiome for wound healing. Our experiments will also generate critical information for clinicians about the prudent use of antibiotics after cleft palate surgery and the effects of depleting the oral microbiome during healing. We will also substantiate the approach of the repletion of the oral commensal bacteria using hydrogel technology as the vehicle to enhance oral wound healing. Together, these studies will yield critical data to inform new therapeutic modalities to lower the prevalence of ONFs following cleft palate surgery.
Selected Publications
-
Toma, Afra I., Julia M. Fuller, Nick J. Willett, and Steven L. Goudy. "Oral Wound Healing Models and Emerging Regenerative Therapies." Translational Research (2021).
-
Amanso, A., Turner, T., Kamalakar, A. et al. Local delivery of FTY720 induces neutrophil activation through chemokine signaling in an oronasal fistula model. Regen. Eng. Transl. Med. (2021). https://doi.org/10.1007/s40883-021-00208-z
-
Amanso, A. M., Kamalakar, A., Bitarafan, S., Abramowicz, S., Drissi, H., Barnett, J. V., ... & Goudy, S. L. (2021). Osteoinductive effect of soluble transforming growth factor beta receptor 3 on human osteoblast lineage. Journal of Cellular Biochemistry, 122(5), 538-548.
- Kamalakar, A., McKinney, J. M., Duron, D. S., Amanso, A. M., Ballestas, S. A., Drissi, H., ... & Goudy, S. L. (2021). JAGGED1 stimulates cranial neural crest cell osteoblast commitment pathways and bone regeneration independent of canonical NOTCH signaling. Bone, 143, 115657.
- Ballestas, S. A., Turner, T. C., Kamalakar, A., Stephenson, Y. C., Willett, N. J., Goudy, S. L., & Botchwey, E. A. (2019). Improving hard palate wound healing using immune modulatory autotherapies. Acta biomaterialia, 91, 209-219.
- Kamalakar A, Oh MS, Stephenson YC, Ballestas-Naissir SA, Davis ME, Willett NJ, Drissi HM, Goudy SL. A non-canonical JAGGED1 signal to JAK2 mediates osteoblast commitment in cranial neural crest cells. Cell Signal. 2019 Feb;54:130-138. doi: 10.1016/j.cellsig.2018.12.002. Epub 2018 Dec 8. PubMed PMID: 30529759.
- Farrell AN, Landry AM, Yee ME, Leu RM, Goudy SL. Sensorineural hearing loss in children with sickle cell disease. Int J Pediatr Otorhinolaryngol. 2018 Dec 5;118:110-114. doi: 10.1016/j.ijporl.2018.12.002. [Epub ahead of print] PubMed PMID: 30599285.
- Goudy SL. Head and Neck Pathology and Pathophysiology in Neonates and Children from the Otolaryngologist Perspective. Clin Perinatol. 2018 Dec;45(4):xix-xx. doi: 10.1016/j.clp.2018.09.001. Epub 2018 Sep 28. PubMed PMID: 30396421.
- Dao AM, Goudy SL. Cleft Palate Repair, Gingivoperiosteoplasty, and Alveolar Bone Grafting. Facial Plast Surg Clin North Am. 2016 Nov;24(4):467-476. doi: 10.1016/j.fsc.2016.06.005. Review. PubMed PMID: 27712814.
- Hill CR, Jacobs BH, Brown CB, Barnett JV, Goudy SL. Type III transforming growth factor beta receptor regulates vascular and osteoblast development during palatogenesis. Dev Dyn. 2015 Feb;244(2):122-33. doi: 10.1002/dvdy.24225. Epub 2014 Dec 1. PubMed PMID: 25382630; PubMed Central PMCID: PMC4310801.
- Crockett DJ, Goudy SL. Cleft lip and palate. Facial Plast Surg Clin North Am. 2014 Nov;22(4):573-86. doi: 10.1016/j.fsc.2014.07.002. Epub 2014 Aug 24. Review. PubMed PMID: 25444729.
- Hill CR, Yuasa M, Schoenecker J, Goudy SL. Jagged1 is essential for osteoblast development during maxillary ossification. Bone. 2014 May;62:10-21. doi: 10.1016/j.bone.2014.01.019. Epub 2014 Feb 1. PubMed PMID: 24491691; PubMed Central PMCID: PMC4306467.
- Mankarious LA, Goudy SL. Craniofacial and upper airway development. Paediatr Respir Rev. 2010 Dec;11(4):193-8. doi: 10.1016/j.prrv.2010.06.003. Epub 2010 Aug 7. Review. PubMed PMID: 21109176.
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, Murray JC, Schutte BC. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6). Nat Genet. 2006 Nov;38(11):1335-40. Epub 2006 Oct 15. PubMed PMID: 17041601; PubMed Central PMCID: PMC2082114.