Our laboratory investigates the mechanisms of entry and fusion of enveloped viruses, including HIV-1, Influenza virus, arenaviruses, coronaviruses, and others. We use functional assays and real-time virus tracking to delineate the entry and fusion steps of infection of diverse viruses. We visualize the release of single virus content into the cytoplasm, and subsequent viral core trafficking and uncoating that culminate in infection. We are also interested in understanding the role of multiple HIV-1 capsid-interacting host factors in early steps of infection. A major focus in the lab is on uncovering the mechanisms of antiviral activity of host restriction factors that target virus entry/fusion, such as IFITM3 and SERINC5. In conjunction with live cell imaging, we employ super-resolution microscopy and correlative light-electron microscopy (CLEM) techniques to delineate structural intermediates of virus entry.
https://youtu.be/b-tZWZ4vnlI
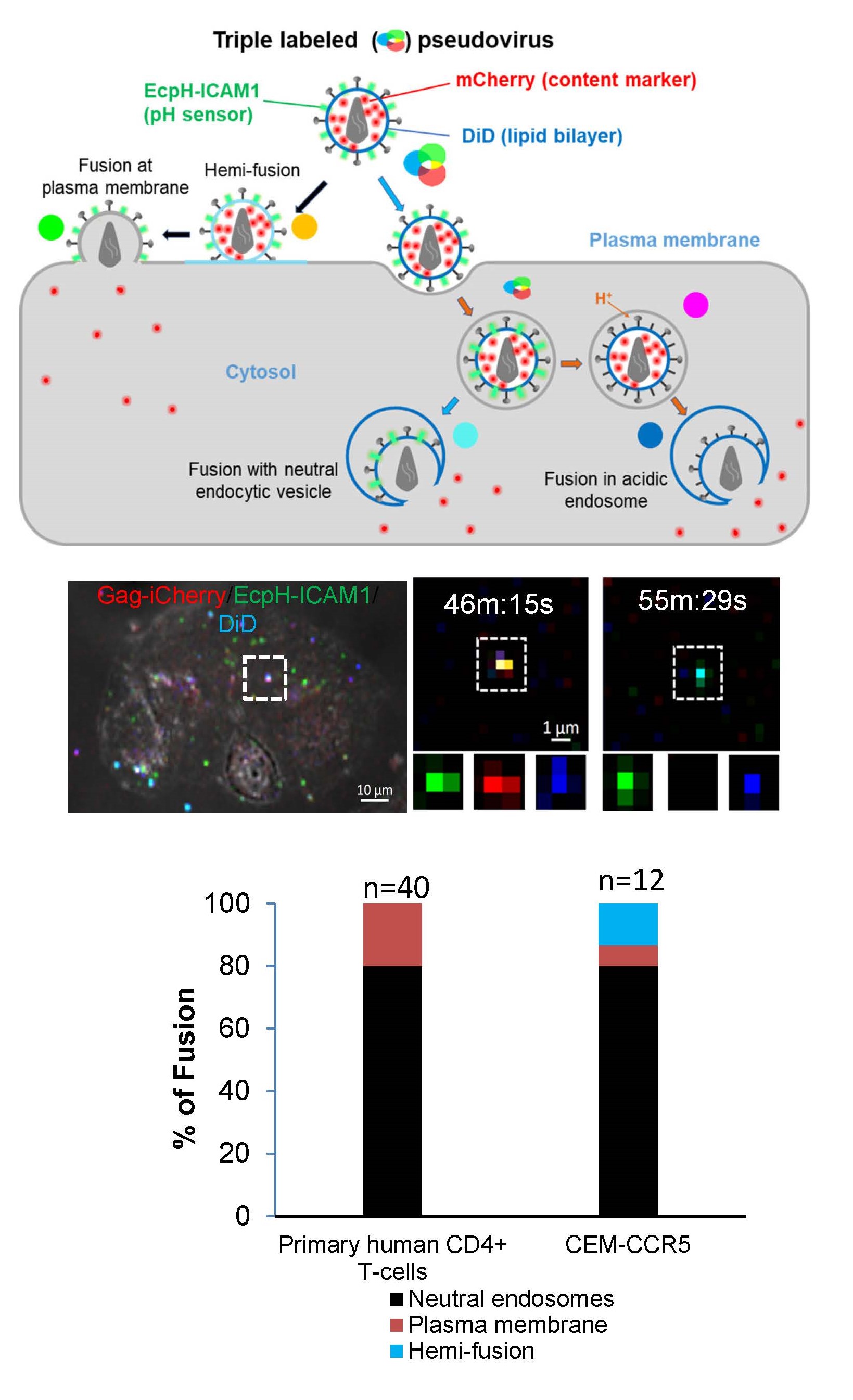
Our single virus tracking experiments revealed that HIV-1 preferentially fuses with pH-neutral intracellular vesicles but not with the cell plasma membrane of primary CD4+ T-cells. This was achieved through co-labeling of HIV-1 pseudoviruses with three fluorescent tags: viral content marker released upon fusion, virus surface-exposed pH sensor, and a lipophilic dye (Figure 1). The extent of DiD dilution upon fusion discriminates between HIV-1 fusion with the plasma membrane vs endosomes. The extent of lipophilic dye dilution upon viral fusion with the plasma membrane and the endosomal membrane allows pinpointing the sites of HIV-1 hemifusion/fusion (Movie 1). We are interested in delineating the mechanism that favors HIV-1 fusion with early endocytic compartments.
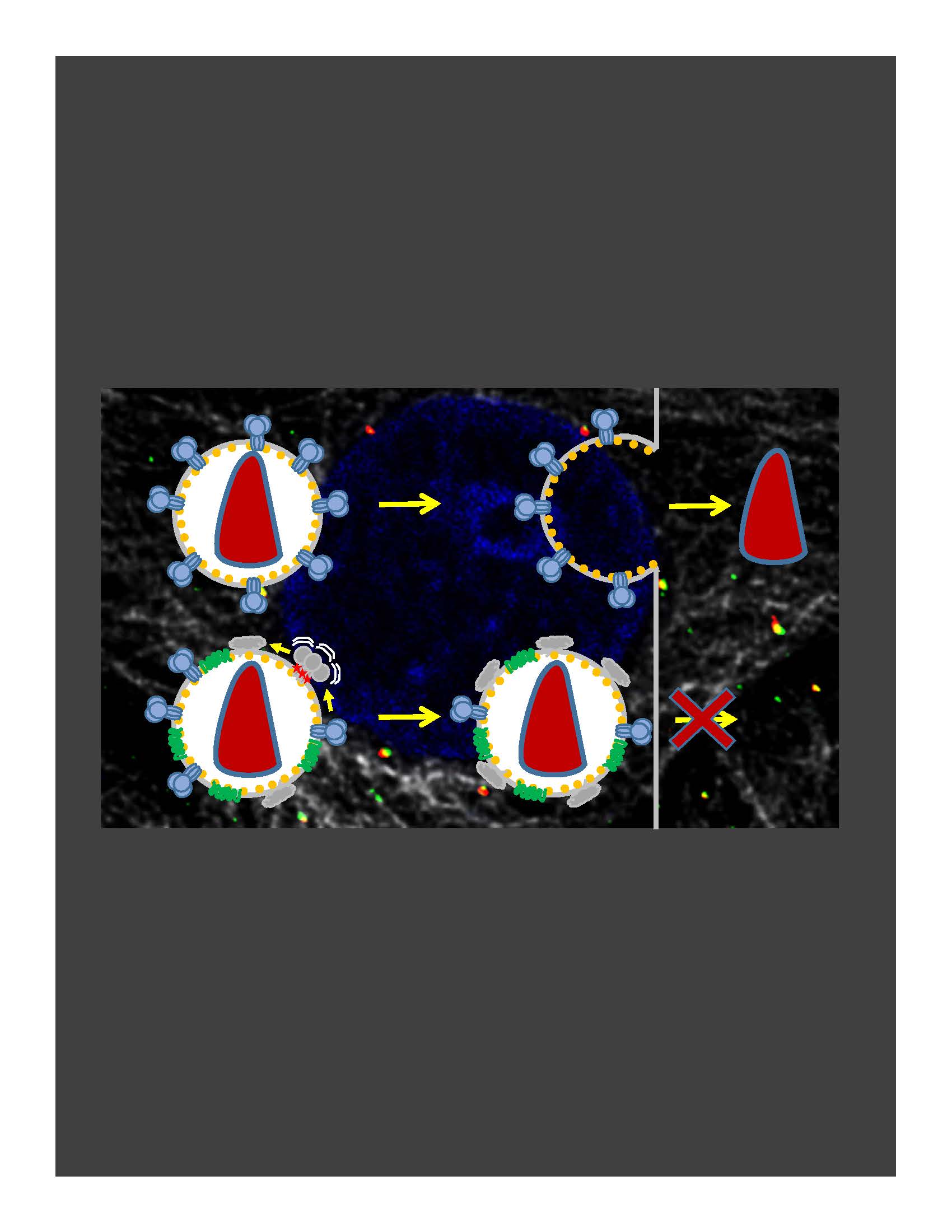
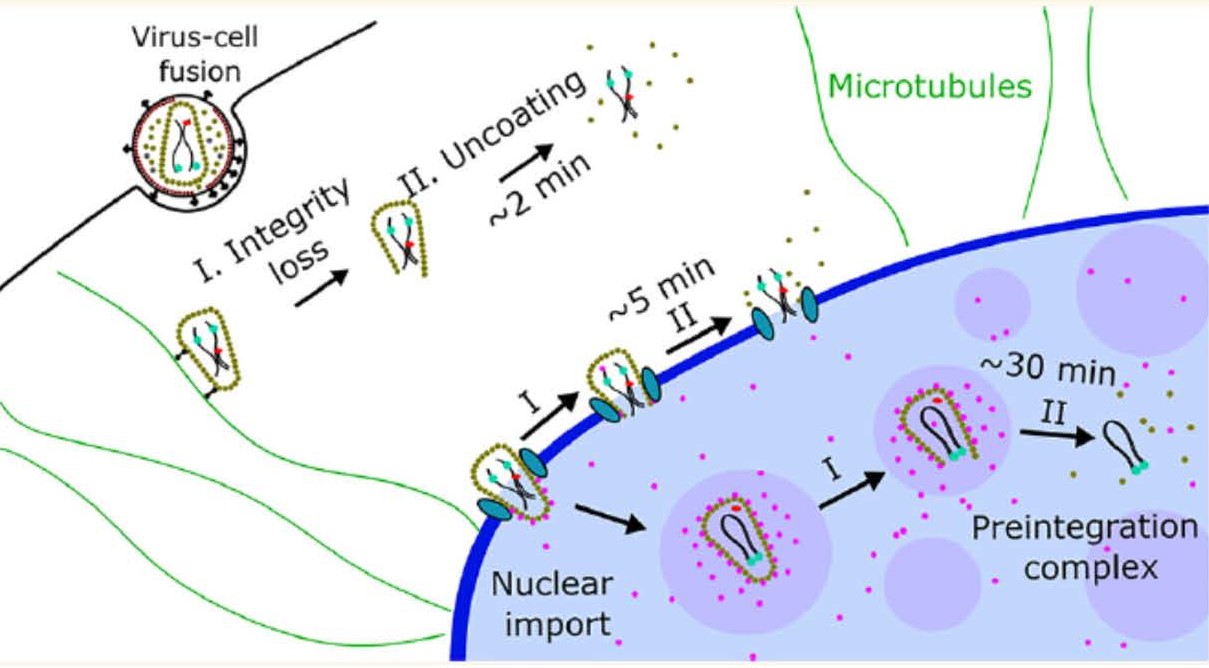
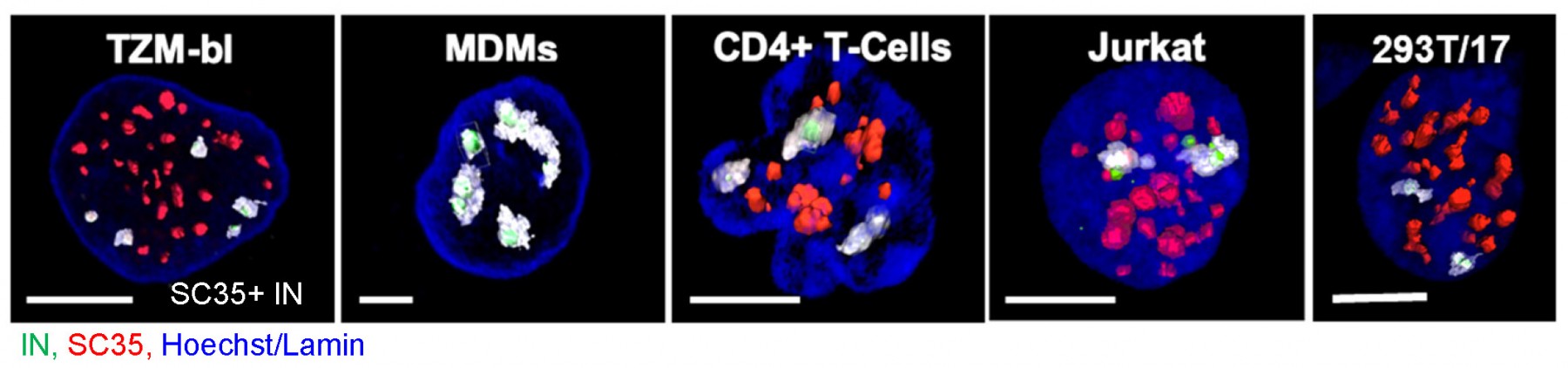
Timely disassembly of the cone-shaped HIV-1 capsid/core (uncoating) after virus-cell fusion is a prerequisite for establishing infection. We have visualized HIV-1 uncoating by indirectly labeling the viral capsid with CypA-DsRed and directly labeling through amber codon suppression. Co-labeling click-labeled CA protein with organic dyes and trapping a fluid-phase marker within the core enables monitoring the uncoating intermediates, from small defect formation to core disassembly (Figure 2 and Movie 2). This co-labeling approach revealed intact HIV-1 cores entering and uncoating in the nucleus. We also found that viral pre-integration complexes are transported to nuclear speckles in macrophages and other cell types where they integrate into speckle-associated genomic domains, SPADs.
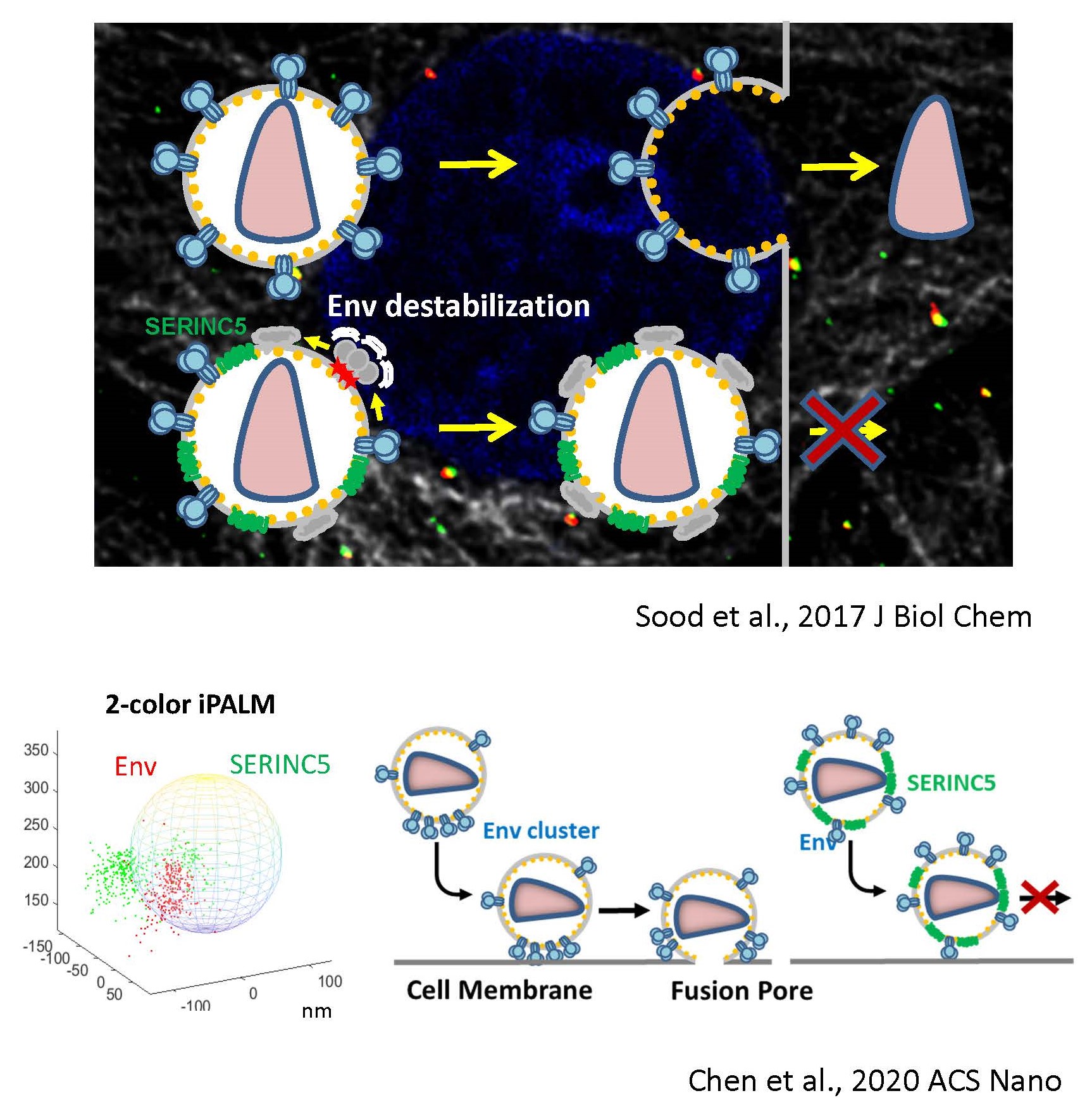
In the absence of HIV/SIV Nef expression, SERINC5 incorporates into budding virions and inhibits viral fusion, but the mechanism of restriction remains unclear. Envelope glycoproteins (Env) from different HIV-1 isolates exhibit a broad range of sensitivity to SERINC5. We have shown that SERINC5 incorporation alters Env conformation and sensitizes Env to neutralizing antibodies and fusion inhibitors. We also found that SERINC5 accelerates spontaneous inactivation of Env on virions (Figure 3). Using 2D- and 3D-superresolution imaging, we have observed that SERINC5 tends to disrupt functional Env clusters on mature HIV-1 particles. We have also implemented an assay to measure the transmembrane lipid asymmetry in single virions that allows to correlate SERINC5’s lipid scramblase activity to HIV-1 restriction. We are also interested in delineating the mechanism of HIV-1 restriction by the interferon-inducible host factor, MX2, that binds to viral capsid.
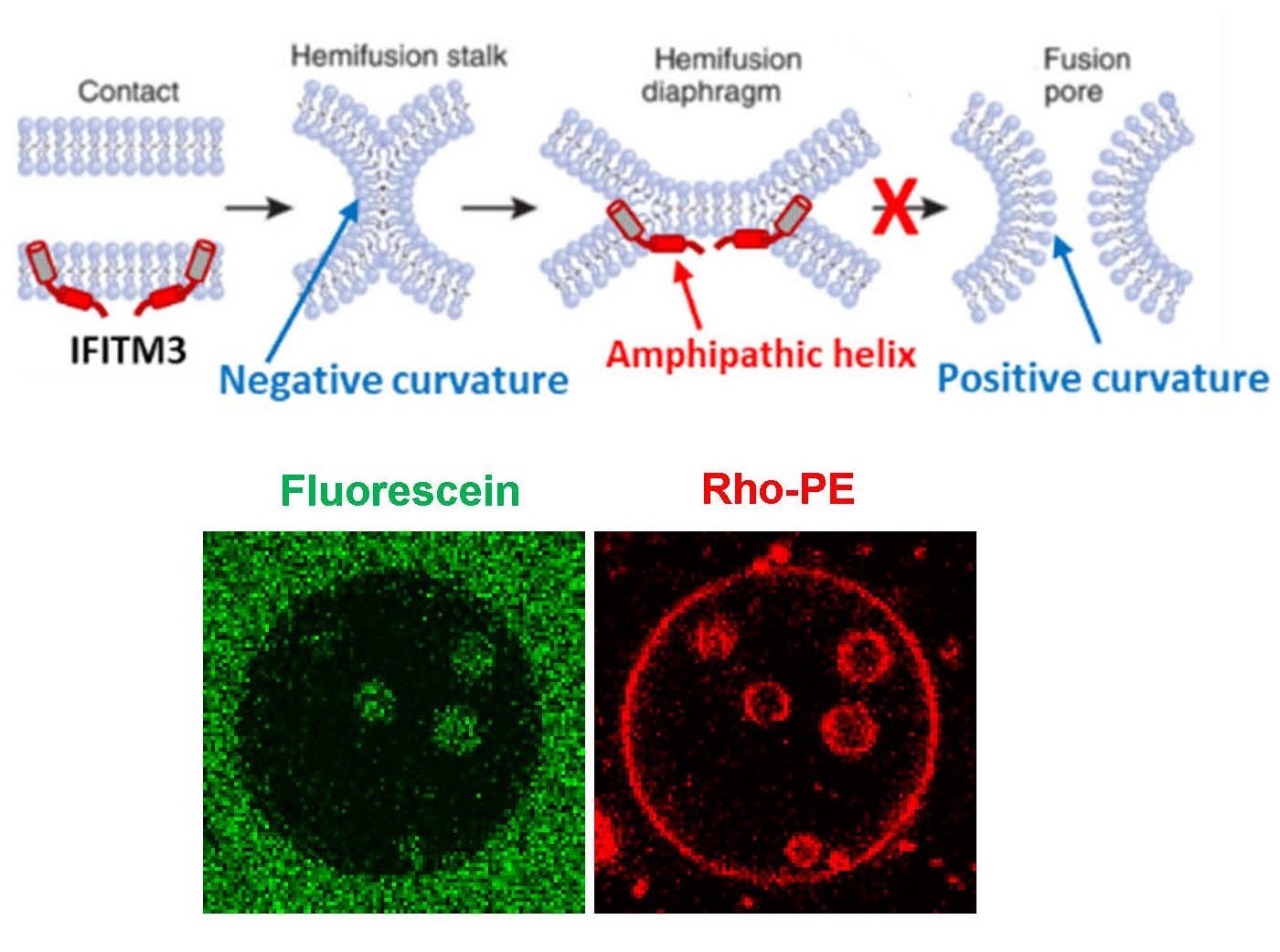
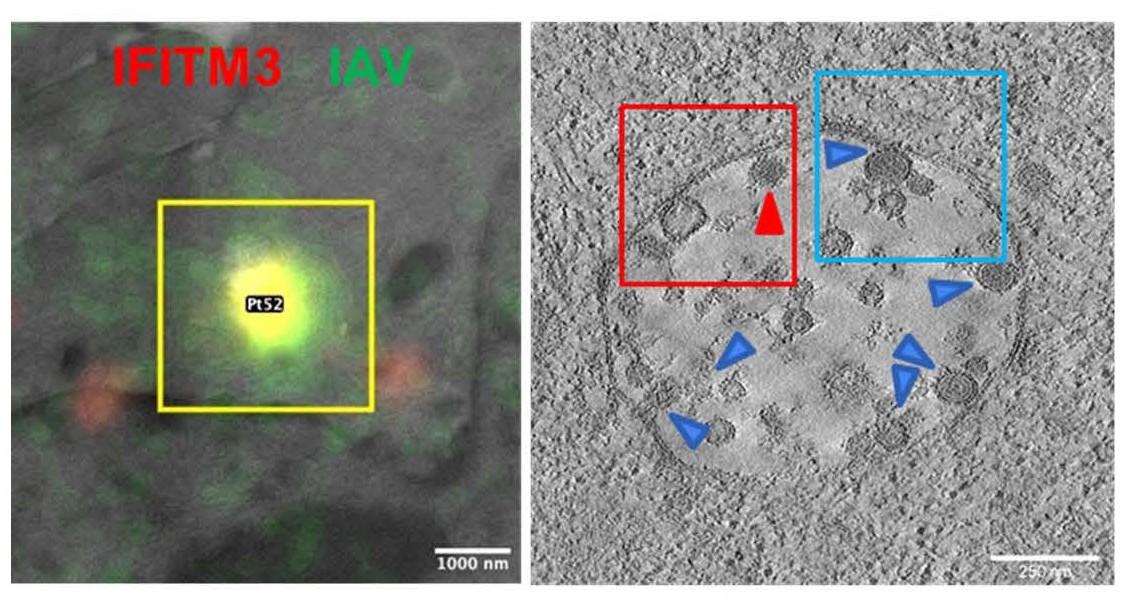
IFITMs inhibit infection of diverse enveloped viruses, including the Influenza A virus, West Nile, Ebola and others. Using single virus imaging, we have shown that IFITM3 concentrates at the sites of Influenza virus entry and allows lipid mixing but abrogates the release of viral content into the cytoplasm (Figure 4, Movies 3 and 4). We also found that arenaviruses, which are resistant to IFITM restriction, can traffic and fuse with endosomal compartments devoid of IFITM3. We have shown that IFITM3 imposes negative curvature and increases rigidity of the cytoplasmic leaflet of a target membrane, thereby disfavoring the formation of fusion pores and trapping virions at a hemifusion intermediate. We are currently employing correlative light-electron microscopy (CLEM, Figure 5) approaches to study IFITM-mediated virus restriction and its antagonism by cyclosporin A.
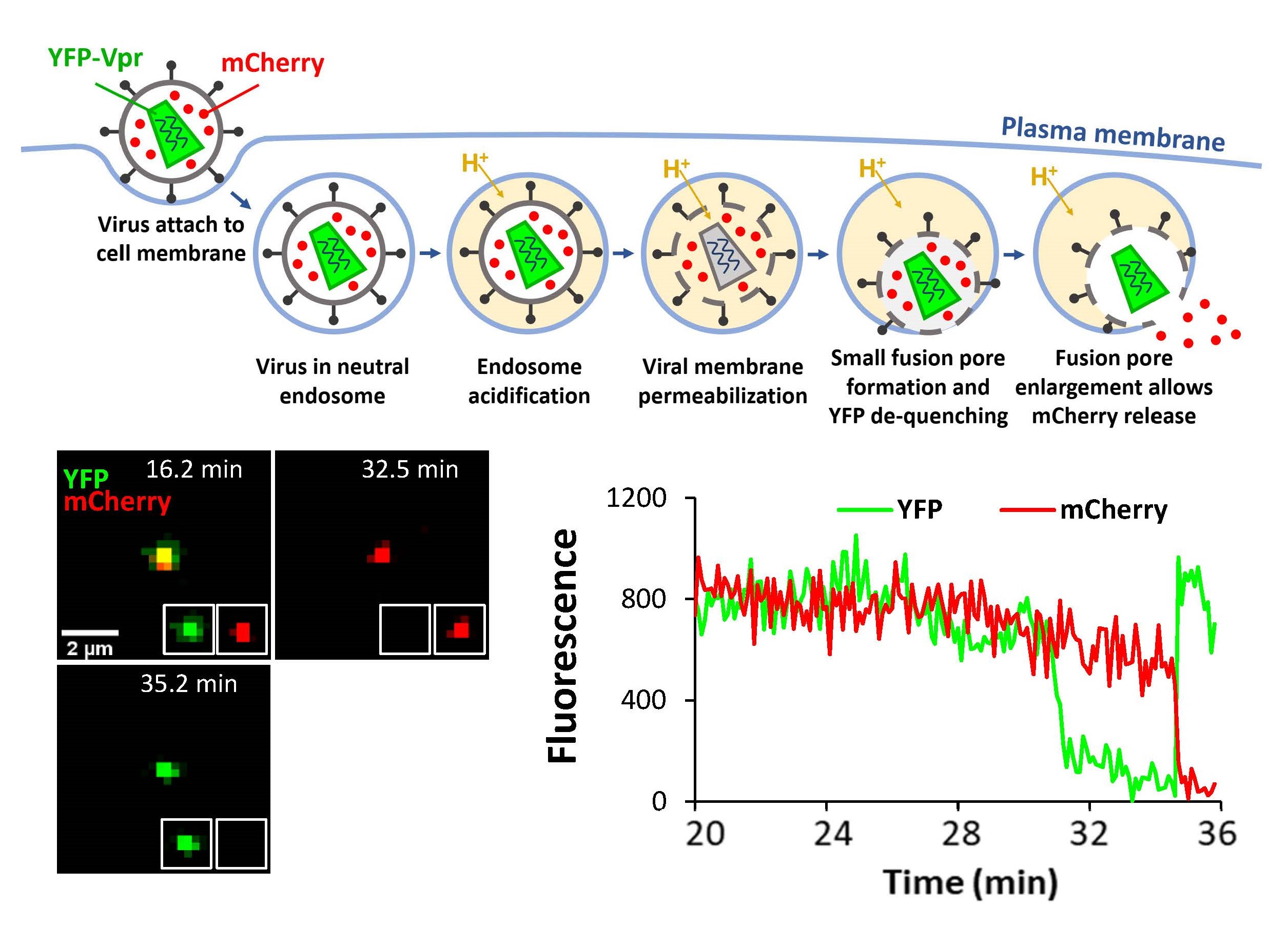
Old and New World arenaviruses can cause severe hemorrhagic fever in humans. There are currently no FDA approved vaccines or drugs to battle arenavirus infection. The mechanism of arenavirus-cell fusion mediated by the glycoprotein complex (GPC) is not well understood. Using single virus labeling and imaging tools to examine entry pathways and the fusion mechanism of arenaviruses, such as Lassa, LCMV and Junin viruses. Our data suggest that arenavirus fusion is uniquely preceded by a viral membrane permeabilization step (Figure 6 and Movie 5), which likely promotes subsequent uncoating of arenaviral core. We also discovered that the late endosome-resident lipid, BMP, specifically promotes late steps of arenavirus fusion. We are also working on defining the preferred sites of SARS-CoV-2 fusion in cells expressing or lacking the TMPRSS2 protease and the impact of IFITM expression using single pseudovirus tracking in living cells.
http://www.ncbi.nlm.nih.gov/sites/myncbi/gregory.melikian.1/bibliography/40733965/public/?sort=date&direction=descending
Yinglin Li
Levi Gifford
Manish Sharma
Andrew Ten Eyk
Yi-Han Lu
Manav Kumar
Yen-Cheng Chen
Matthew Prellberg
Xiangyang Guo
Ashwanth Francis
Satya Singh
Kristina Rowland
Christine Lee
Junghwa Choi-Kirschman
Caleb Mason
Chetan Sood
Krishna Suddala
Charline Giroud
Tanay Desai
Jennifer Spence
Yoon-Hyeun Oum
Lusine Demirkhanyan
Naoyuki Kondo
Kiran Verma
Michelle de la Vega
Sergi Padilla-Parra
Surbhi Jain
Rolf Suter
Nishi Sharma
Kosuke Miyauchi
Olga Latinovic
Yuri Kim
Grant Jones
Gennadiy Novitskiy
Naveen Jha
Patrick Millet
Victoria Saakian
Vladimir Morozov
Sergey Cheresiz
Michael Leung
Levon Abrahamyan
Visiting students/scholars/volunteers:
Nivriti Galhaut, David Gevorgyan, Pedro Matos, Margaret Black, Ani Avetissyan
.jpg)