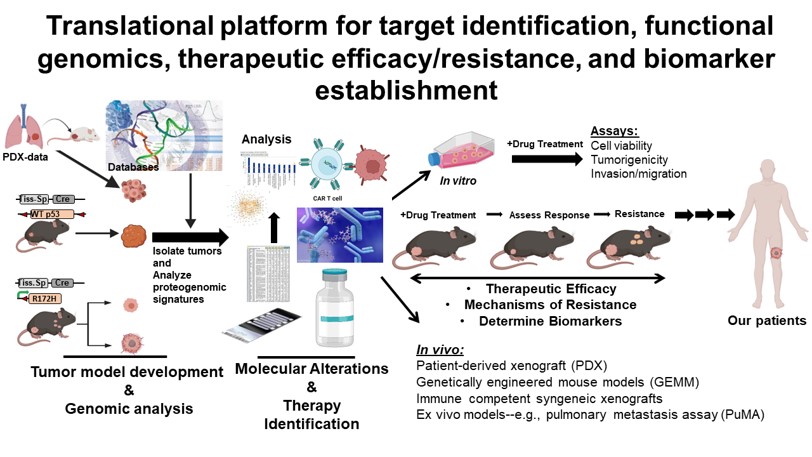
Overview of Research
Due to the relatively inferior long-term patient outcomes, we recognize the need for improving our understanding of pediatric sarcomas, specifically osteosarcoma, Ewing sarcoma and rhabdomyosarcoma. The laboratory has tremendous interest and experience in merging innovative murine models and patient-derived resources towards garnering molecular insights into sarcoma initiation, development, metastatic progression, and therapeutic resistance and translating these findings towards testing novel therapeutic interventions.
Research in our laboratory is focused on these areas:
- Utilizing tissue-specific genetic perturbation to form novel, applicable transgenic mouse models of metastatic osteosarcoma and rhabdomyosarcoma, both conditions that have particularly poor patient outcomes. Such models allow for further understanding of critical genetic and proteomic alterations involved in sarcoma development, progression and metastasis and can be utilized as preclinical models for therapeutic testing and efficacy.
- Use of innovative in vitro, ex vivo and in vivo models to investigate the roles of critical signal transduction mechanisms, such as the Wnt-signaling pathway, in the metastatic and therapeutic resistant conditions.
- Investigating the role and the therapeutic potential of the tumor microenvironment, focusing on non-tumor cell contributions and metabolic alterations, towards sarcoma initiation, development, and progression.
We have developed multiple local, national, and international academic collaborations to further enhance our studies from a basic science and translational/clinical approach. In addition, we have formed collaborations with multiple pharmaceutical companies to test the efficacy of novel therapeutic agents on pediatric sarcomas, which have led to the design and implementation of new clinical trials for pediatric sarcoma patients.

Taku Yamamichi, MD, PhD, Post-Doctoral Fellow
Education: MD, PhD (Medicine); Graduate School of Medicine, Osaka University
Current Project: Mechanisms of metastasis in pedatric sarcomas
taku.yamamichi@emory.edu

Bikesh Kumar Nirala, PhD, Associate Research Scientist
Education: PhD (Biomedical Engineering), Indian Institute of Technology
Current Project: Development and characterization of the osteosarcoma mouse model
bikesh.kumar.nirala@emory.edu

Tajhal D. Patel, PhD, Post Doctoral Fellow
Education: PhD (Cell and Molecular Biology), Baylor College of Medicine
Current Project: Utilizing bioinformatic approaches to study pediatric sarcomas
dayaram@bcm.edu

Juan Dou, MD, MPH, Senior Research Specialist
Education: Masters (Philosophy in Pediatrics), Sun Yat-sen University of the third affiliated hospital, Guangzhou, China, 2006; MD (Medical Sciences), Wannan Medical College, Wuhu, Anhui, China, 1994
Current Project: Molecular Mechanisms underlying Rhabdomyosarcoma (RMS) and Osteosarcoma (OS) and Mechanism-based therapeutics for these children-associated solid tumors
jdou2@emory.edu

Sarah S. Kappa, MD, Instructor of Pediatrics (Texas Children's Hospital)
Education: MD, Emory University School of Medicine; Residency and Chief Residency, Children's National Hospital, Washington DC; Fellowship, Texas Children's Hospital/Baylor College of Medicine, Houston, TX
Current Project: Investigating Oncogenic Signaling Inhibition in Combination with Immune Checkpoint Blockade for the Treatment of Osteosarcoma
sskappa@texaschildrens.org

Heath Bradley, MS, Senior Research Specialist
Education: BS (Molecular Biology), Grove City College; MS (Biotechnology), Johns Hopkins University
hbradle@emory.edu

Isabel Petrescu, MS, Graduate Student, GMB Program
Education: BS (Genomics and Molecular Genetics), Michigan State University; MS (Mircobiology and Molecular Genetics), Michigan State University
isabel.petrescu@emory.edu

Sukjoo Cho, MD, Pediatric Hematology/Oncology Fellow
Education: MD, Hallym University; Residency (Pediatrics), University of South Florida; Fellowship, Emory University/Children's Healthcare of Atlanta
sukjoo.cho@emory.edu

Katherine Shelmidine, Graduate Student, Cancer Biology Program
Education: BA (Biochemistry and Molecular Biology), Wooster College
katherine.shelmidine@emory.edu

Megan Noh
Education: Emory University (Neuroscience and Behavioral Biology), Class of 2025
megan.noh@emory.edu

Erin Yoon
Education: Emory University (Human Health), Class of 2026
erin.yoon@emory.edu

Ilsa Quereshi
Education: Emory University (Chemistry and Psychology), Class of 2026
ilsa.quereshi@emory.edu
Liikanen I, Lauhan C, Quon S, Omilusik K, Phan AT, Bartrolí LB, Ferry A, Goulding J, Chen J, Scott-Browne JP, Yustein JT, Scharping NE, Witherden DA, Goldrath AW. Hypoxia-inducible factor activity promotes antitumor effector function and tissue residency by CD8+ T cells. J Clin Invest. 2021 Apr 1;131(7):e143729. doi: 10.1172/JCI143729. PMID: 33792560; PMCID: PMC8011896.
Nakahata K, Simons B, Pozzo E, Shuck R, Kurenbekova L, Patel T, Yustein JT. MyoD-Cre driven alterations in mutant Kras and p53 leads to a mouse model with histological and molecular characteristics of human rhabdomyosarcoma with pre-clinical applications. Dis Model Mech. 2022 Feb 1.15(2):dmm049004. doi: 10.1242/dmm.049004. Epub 2022 Feb 17.PMID: 35174853 Editor’s Choice; Highlighted in special Ras Issue due out in 02/2022.
Tu J, Huo Z, Yu Y, Zhu D, Xu A, Huang MF, Hu R, Wang R, Gingold JA, Chen YH, Tsai KL, Forcioli-Conti NR, Huang SXL, Webb TR, Su J, Bazer DA, Jia P, Yustein JT, Wang LL, Hung MC, Zhao Z, Huff CD, Shen J, Zhao R, Lee DF. Hereditary retinoblastoma iPSC model reveals aberrant spliceosome function driving bone malignancies. Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2117857119. doi: 10.1073/pnas.2117857119. Epub 2022 Apr 11. PMID: 35412907; PMCID: PMC9169787.
Guo A, Huang H, Zhu Z, Chen MJ, Shi H, Yuan S, Sharma P, Connelly JP, Liedmann S, Dhungana Y, Li Z, Haydar D, Yang M, Beere H, Yustein JT, DeRenzo C, Pruett-Miller SM, Crawford JC, Krenciute G, Roberts CWM, Chi H, Green DR. cBAF complex components and MYC cooperate early in CD8+ T cell fate. Nature. 2022 Jul;607(7917):135-141. doi: 10.1038/s41586-022-04849-0. Epub 2022 Jun 22. PMID: 35732731; PMCID: PMC9623036.
Tsang SV, Rainusso N, Liu M, Nomura M, Patel TD, Nakahata K, Kim HR, Huang S, Rajapakshe K, Coarfa C, Man TK, Rao PH, Yustein JT. LncRNA PVT-1 promotes osteosarcoma cancer stem-like properties through direct interaction with TRIM28 and TSC2 ubiquitination. Oncogene. 2022 Dec;41(50):5373-5384. doi: 10.1038/s41388-022-02538-w. Epub 2022 Nov 8. PMID: 36348010.
Subasinghe SAAS, Ortiz CJ, Romero J, Ward CL, Sertage AG, Kurenbekova L, Yustein JT, Pautler RG, Allen MJ. Toward quantification of hypoxia using fluorinated EuII/III-containing ratiometric probes. Proc Natl Acad Sci U S A. 2023 Apr 11;120(15):e2220891120. doi: 10.1073/pnas.2220891120. Epub 2023 Apr 5. PMID: 37018203; PMCID: PMC10104500.
Nirala BK, Patel TD, Kurenbekova L, Shuck R, Dasgupta A, Rainusso N, Coarfa C, Yustein JT. MYC regulates CSF1 expression via microRNA 17/20a to modulate tumor-associated macrophages in osteosarcoma. JCI Insight. 2023 Jul 10;8(13):e164947. doi: 10.1172/jci.insight.164947. PMID: 37279073; PMCID: PMC10371352.
NIH/R21 - 01/2022-12/2024
Dissecting and Targeting the Role of Galnt14 in High-Risk Osteosarcoma
Goals: This study will lead to the identification of critical candidate therapeutic modalities for the treatment of high-risk OS
Role: PI
MPI: Kohler
Peach Bowl LegACy Fund - 07/01/2023-06/30/2026
A Phase 1b Study of Tegavivint,a TBL1 inhibitor, with Gemcitabine in Children, Adolescents, and Young Adults with Relapsed or Refractory Osteosarcoma
Goals: 1. Define the maximum tolerated dose and/or recommended phase 2 dose of Tegavivint in combination with gemcitabine. 2. Describe toxicities, in pediatic and AYA patients with relapsed or refractory osteosarcoma
Role: PI
MPI: Cash
MIB (Making it Better) Research Award - 08/2022-07/2024
Combinatorial therapies to improve immune-mediated approaches for osteosarcoma
Goals: 1. Assess the efficacy of targeting osteosarcoma development and progression with single agent targeted therapy and combination checkpoint inhibition on murine osteosarcoma models. 2. Assessment of cellular tumor evolution secondary to combinatorial targeted therapies on high-risk OS
Role: PI
Osteosarcoma Institute - 01/2023-12/2024
Use of Combinatorial Therapies to Improve Immune-Mediated Approaches for High-Risk Osteosarcoma
Goals: 1. Evaluate the antitumor activity and characterize the molecular and cellular responses to ICI and characterize the molecular and cellular landscape of OS tumors post-treatment with targeted therapy in our GEMM OS models. 2. Evaluate the antitumor activity and characterize the molecular and cellular responses to single agent vs combinatorial B7-H3-CAR T cell and targeted therapy in murine OS models
Role: PI
NIH/R01 - 12/2022-11/2027
Dissecting and Targeting Oncogenic Functions of PAK4 in High-Risk Rhabdomyosarcoma
Goals: Pre-clinical evaluation of PAK4 inhibition as rational therapeutic approach for the treatment of high-risk RMS. We will examine PAK4’s role as a novel regulator of AS and PAK4-mediated downstream transcriptomic and proteomic signatures that contribute to RMS progression through the use of our extensive inventory of highly relevant human and syngeneic murine pre-clinical models.
Role: PI
The Charlie Landers Foundation - 07/2023-06/2024
Dissecting and Targeting PAK4-Mediated Signaling in Ewing Sarcoma Development and Metastasis
Goals: 1. Pre-clinical evaluation of combination therapy incorporating PAK1 and PAK4 inhibition as a therapeutic approach for the treatment of high-risk Ewing sarcoma. 2. Defining the PAK4-proteo-transcriptomic signaling networks in primary and metastatic Ewing sarcoma
Role: PI
Rutledge Cancer Foundation - 08/2023-07/2024
Testing myr5A encapsulated cytotoxic compounds in xenograft models of Ewing sarcoma
Goals: Assessment of the efficacy of Myr5a targeted delivery of anti-tumor agents for the treatment of Ewing sarcoma. We will perform in vivo studies using our xenograft models of Ewing sarcoma.
Role: PI
DOMPE FARMACEUTICI - 01/2024-01/2025
Targeting CXCR1/2 in metastatic Ewing’s Sarcoma
Goals: In vitro testing of Ladarixin on Ewing sarcoma cells and In vivo testing of Ladarixin on Ewing sarcoma tumor development and progression using orthotopic model
Role: PI






