2024 Pediatric Research Alliance Pilots
Children's and Emory offer pilot grants for faculty and centers in the Pediatric Research Alliance. These pilots are designed to stimulate new research projects, build new collaborations, and increase extramural funding for pediatric research.
PURPOSE: To stimulate new research projects, build new collaborations, and increase extramural funding for pediatric research. Proposals should be aimed towards generating preliminary data for subsequent extramural grant applications.
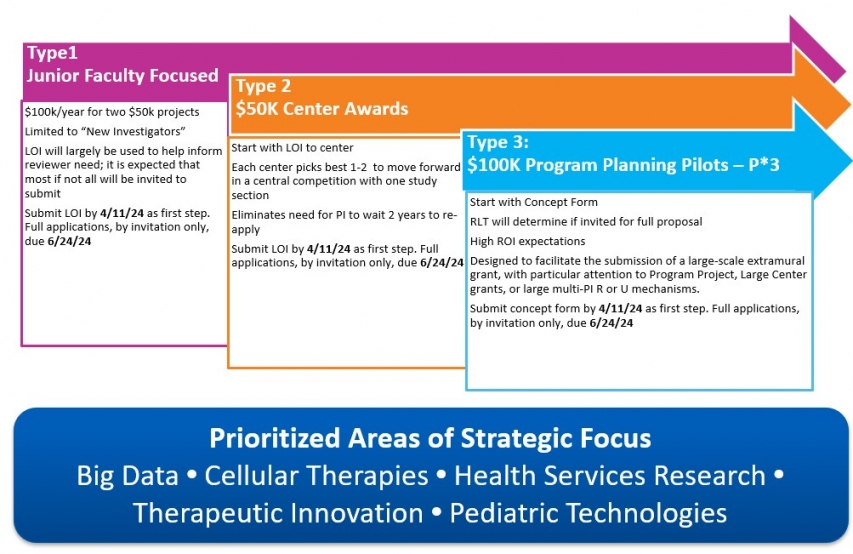
TYPES & AMOUNTS: Three types of awards are available:
- Type 1: Junior Faculty Focused (JFF) Award - $50,000 per award
- Type 2: Center Award - $50,000 per award
- Type 3: Program Planning Pilot (P*3) Awards - up to $100,000 per award
PROJECT PERIOD: October 1, 2024 - September 30, 2025
A PDF version of the RFA can be found here.
Description
These grant opportunities are designed to prepare junior faculty to move from mentored research to independent researcher status. Each award is $50,000.
Eligibility Requirements
- The principal investigator (PI) must have a current rank of Instructor or Assistant Professor in the Emory Department of Pediatrics or a member of the Peds Institute and be no more than 7 years into their faculty appointment.
- Please note that adjunct faculty appointments do not meet this requirement.
- The PI must have NIH New Investigator status, meaning they have not previously competed successfully as PD/PI for a substantial NIH independent research award such as an R01. Click here for a detailed definition and exclusions.
- The PI cannot be PI on any grant with more than $200,000 of direct costs per year.
- The PI must be on a trajectory to become independently funded, meaning they expect to successfully compete as PI for K or R level funding within the next three years. Submitting an extramural grant proposal (of any level) as PI within 1 year of the end date of a funded pilot is a firm requirement.
- The application must include a mentor and a mentee, as with an NIH K career development award.
- The mentee serves as PI of the application.
- The mentor fulfills the requirement for a second researcher.
- The mentor must include a statement in the application on the applicant’s likelihood of being successful as an independent researcher.
- Both the scientific plan and the candidate’s past and future productivity will be factored into the funding decision.
- The applicant’s career objectives must be clearly stated on their biosketch.
Application Process
Letters of Intent (LOIs) are required and must be submitted by April 11th, 2024 at 11:59 pm
LOIs may be a maximum of 2 pages in PDF format (not including references), and must include:
- Project title
- PI and key personnel
- Summary of project and methods (including hypotheses)
- Specify the type and timeline for the extramural grant submission being planned that will be submitted as a result of the funded pilot
- A third page devoted to Specific Aims
- For these JFF LOI’s only, a brief explanation of how the project will contribute to your research career development.
The LOIs will be evaluated based on the feasibility of the proposed science leading to the development of the applicant’s research career.
See the How to Apply tab of this RFA for links to the LOI submission portals.
If a Letter of Intent is selected to move forward to the full application stage, the PI will receive a link before May 20th to submit a full application which will be due on June 24th.
The primary component of all full applications is the research plan: a 4-page, single-spaced, Arial 11 font, .5" margin scientific plan document. Note that references should be listed but are not included in the page limit and that the Specific Aims page is also not included in that page limit. The research plan must include the following components:
- Title
- Background and Significance to Child Health
- Specific Aims (separate from the 4-page document)
- Experimental Design and Methods
- Expected Results
- Timeline of extramural grant submission
JFF applicants (upon invitation) also must include the following components:
- Career Development Plan (max. 2 pages) - Please use this Career Development Plan template.
- Letter(s) of Support from Mentor(s) (max. 2 pages each) - Describe the training and research career development plan for your mentee (i.e., the PI for the JFF). The letter(s) should specifically cover:
- Nature and extent of supervision and mentoring, including the specific resources the mentor will provide to enable the PI to complete the project
- Commitment to PI development during the award period
- The portion of time the PI has available for research
- How this project will facilitate the PIs transition from a mentored to an independent researcher
- All mentor letters must also include the following statements - please copy and paste these three bullets into the mentor letter(s):
- I have read and approved your complete research plan, career development plan and biosketch.
- The project is feasible.
- The project does not overlap with my own funded research.
Additional documents required for all applications include:
- NIH-format Biosketches for all key personnel, including the mentor. Instructions for the current format that must be used are available here.
- NIH-format Other Support pages for principal investigators and mentors. Instructions for the current format are available here.
- Budget, budget narrative, and statements of work for consortium institutions (Required budget template is available under the FAQ and templates tab in this RFA).
- Signed Financial Letters of Agreement from the department head of every department included in the pilot budget except the Emory Department of Pediatrics or Childen’s - see template provided in the templates tab of this RFA.
- (As Applicable) Protection of Human Subjects and/or Vertebrate Animal Sections
- (Optional) Letters of Support from collaborators whose involvement is critical to the proposed project
Proposals will be evaluated based on the feasibility of the specific aims and the proposed science leading to the development of the applicant’s research career.
Description
These grant opportunities are designated for all levels of researchers and must go through one of the eligible centers (link below). Each award is $50,000.
Eligibility
- The principal investigator(s) must hold an appointment at their institution(s), which allows them to serve as principal investigator on extramural grant applications.
- All applications must involve two or more investigators, at least one of whom:
- Has a primary faculty appointment in the Emory Department of Pediatrics (DOP) OR
- Is on the professional staff at Children's Healthcare of Atlanta (Children's). This includes those Emory faculty who are outside of the Emory DOP or employed by the Pediatric Institute and who are on Children's professional staff as well as all non-faculty clinicians on Children's professional staff.
- Faculty from GRA-affiliated institutions are permitted to apply, provided the applications include an investigator from Emory DOP or Children’s Healthcare of Atlanta.
- The following centers are eligible for this grant cycle:
- CCIV
- CCTR
- CF-AIR
- CIAG
- GENI
- CCNR
- CORPH
- HeRO
- Marcus Autism Center
Application Process
Letters of Intent (LOIs) are required and must be submitted by April 11th, 2024 at 11:59 pm
The first stage of the process is a Letter of Intent (LOI) addressed to and evaluated by a team for the applicable center. Applicants should consider consulting with the applicable center director prior to submission to craft the most competitive application possible. LOIs may be a maximum of 2 pages (not including references) plus a one-page specific aims page in PDF format, should be addressed to the director(s) of the center to which the PI is applying, and must include the following components:
- Project title
- PI and key personnel
- Summary of project and methods (including hypotheses)
- Specify the type and timeline for the extramural grant submission being planned that will be submitted as a result of the funded pilot
- A third page devoted to Specific Aims
The LOIs will be reviewed based on the following criteria:
- Strength of the overall research plan and rigor of methodology
- Relevance to Child Health
- Relevance to Center goals
- Addressing one or more of our strategic areas of need: Big Data, Cellular Therapies, Health Services Research, Therapeutic Innovation, and Pediatric Technologies. *Note that, while not required, applications that address strategic areas of need will be prioritized
- Rigor of Specific Aims
- Likelihood of resulting in a strong extramural grant application
- Feasibility
Only a select number of LOIs will be moved forward to the full application stage. If a Letter of Intent is selected to move forward, the PI will receive a link to submit a full application no later than May 20th and full applications will be due June 24th.
The primary component of all full applications is the research plan: a 4-page, single-spaced, Arial 11 font, .5" margin scientific plan document. Note that references should be listed but are not included in the page limit and that the Specific Aims page is also not included in that page limit. The research plan must include the following components:
- Title
- Background and Significance to Child Health
- Specific Aims (separate from the 4-page document)
- Experimental Design and Methods
- Timeline for submission of extramural grant proposal
- Expected Results
Additional documents required for all applications include:
- NIH-format Biosketches for all key personnel. All applications must use the current format; instructions are available here.
- NIH-format Other Support pages for principal investigators. Instructions for the current format are available here.
- Budget, budget narrative and statements of work for consortium institutions (Required budget template is available under the FAQ and templates tab in this RFA)
- Signed Financial Letters of Agreement from the department head of every department included in the pilot budget except the Emory Department of Pediatrics of Children’s - see template provided in the templates tab of this RFA.
- (As Applicable) Protection of Human Subjects and/or Vertebrate Animal Sections
- (Optional) Letters of Support from collaborators whose involvement is critical to the proposed project
The Proposals will be reviewed by a centralized review committee based on the following criteria:
- Strength of the overall research plan and rigor of methodology
- Relevance to Emory and Children’s Healthcare of Atlanta’s goals and our strategic areas of need
- Relevance to Child Health
- Rigor of Specific Aims
- Feasibility
Description
The P*3 category is designed to facilitate the submission of a large-scale extramural grant, with particular attention to Program Project, Large Center grants, or large multi-PI R or U mechanisms (note that if it is a large R grant, you will need to reach out to Stacy Heilman to discuss eligibility before submitting). Further, these awards are meant to cover an inter- or multidisciplinary approach to a research topic or to approach a research topic from multiple, but related disciplines and methods. While this pilot award is somewhat flexible in nature, the following guidelines should help contextualize the ways in which such an award might be used to build a strong extramural proposal at the end of the pilot grant period. Each award is for $100,000.
Like the other two pilot categories, the P*3 application process will occur in two stages. However, instead of an LOI, the applicant will submit a concept form in the application portal to allow for administrative and strategic evaluation regarding full proposal invitations.
Eligibility
- The principal investigator(s) must hold an appointment at their institution(s), which allows them to serve as principal investigator on extramural grant applications.
- All applications must involve two or more investigators, at least one of whom:
- Has a primary faculty appointment in the Emory Department of Pediatrics (DOP) OR
- Is on the professional staff at Children's Healthcare of Atlanta (Children's). This includes those Emory faculty who are outside of the Emory DOP or employed by the Pediatric Institute and who are on Children's professional staff as well as all non-faculty clinicians on Children's professional staff.
- Faculty from GRA-affiliated institutions are permitted to apply, provided the applications include an investigator from Emory DOP or Children’s Healthcare of Atlanta.
Application Process
Concept forms are required and must be submitted by April 11th, 2024 at 11:59 pm
All concept forms require the following information:
- Title
- Background and significance to Child Health (300-word limit)
- Affiliation
- Credentials
- Brief justification of each team member’s role and how their expertise is important to the project
- Concrete outline for pursuing extramural funding (300-word limit)
- Goals and structure of the eventual extramural application
- External funder name and grant type/mechanism
- Timeline/deadline for submission
- Specific aims (one page) of the planning grant, including
- Description of the areas of strategic need, if applicable, that would be met: Big Data, Cellular Therapies, Health Services Research, Therapeutic Innovation, Pediatric Technologies
- Indication of what the money would be used for
- Other in kind and/or monetary and resource contributions
- Timeline and milestones of planning grant
Forms will be reviewed by pediatric research leadership according to the following criteria:
- Strength of the plan
- Relevance to child health and our defined prioritized areas of strategic focus (see infographic) *Note that, while not required, applications that address strategic areas of need will be prioritized
- Likelihood of successful extramural funding/path to sustainability
If a concept form is selected to move forward, the corresponding PI will receive a link to submit a full proposal application.
The primary component of all full applications is the research plan: a 4-page, single-spaced, Arial 11 font, .5" margin scientific plan document. Note that references should be listed but are not included in the page limit and that the Specific Aims page is also not included in that page limit. The research plan must include the following components:
- Title
- Background and significance to child health
- Specific aims of the planning grant (separate from the 4-page document)
- Detailed timeline and description of milestones and activities leading up to extramural submission
- Description of planning activities, e.g., project management support plan; retreat; collaborative experiments; shared postdoc or other relevant team member, refining new approaches; techniques; or methodologies; access to a shared resource, technology, or database; protocol development; and protected time for the PI to develop the extramural application (not to exceed 10% effort and must be well justified). This list is meant to provide examples and guidance but is not necessarily exhaustive
- Leadership and team interactions plan (Think of this as the beginnings of an Administrative Core.)
- Timeline for submission of extramural grant proposal
Additional requirements:
- Line-item budget
- Detailed budget justification
An appendix should be included with the following allowable items:
- Required: Key Personnel Biosketches
- Required: Key Personnel Other Support
- Protection of Human Subjects and/or Vertebrate Animal Sections (if applicable)
- Optional: Cited publications
- Optional: Previous collaborative publications and/or other relevant scholarly outputs/outcomes
-
Other documentation as needed based on Concept forms (to be decided by Research leadership)
Full proposals will be reviewed by a study section according to NIH procedures.
All applications must be submitted electronically through the online application portals.
Please make sure you select the correct link; projects cannot be transferred between application portals.
The principal investigator(s) must:
- Submit a progress report within one month of the end date of the pilot project in the format specified in the Notice of Award.
- Submit a related application for extramural funding within one year of the end date of the pilot project.
- Present the progress and results of your project as requested by the center director.
- Submit an abstract and provide an oral or poster presentation at the annual Southeastern Pediatric Research Conference.
- Submit an annual update report in an online grants management system in response to requests/prompts from staff for up to six years after the end date of the pilot project. This report must include publications, extramural grant applications and awards, press releases, conference presentations, invention disclosures, patents (pending and approved), and devices or technology that resulted from the pilot.
- Funds may not be used to build databases or other infrastructure for types 1 and 2.
- Investigators may not receive funding from a Pediatric Research Center or the Pediatric Research Alliance for multiple projects if the projects have the same or overlapping aims.
- If you received a Pediatric Research Center pilot grant in the past and are not in compliance with the requirements of your previous award, you are not eligible to apply for another pilot grant from the Pediatric Research Alliance. All pilots awarded in 2011 or later required the PI to submit a proposal for extramural funding within one year of the end date of the pilot project. Unless no cost extensions were granted, pilots awarded between 2011 and 2019 should have concluded by 6/30/2020 and should lead to submission of an extramural research proposal by 6/30/2021. If you are not sure if you are in compliance, please contact Julie Hawk to confirm your eligibility. To request a waiver of the extramural funding application requirement, please click here.