CCTR MISSION
To foster excellence, growth, mentorship, operational efficiency and innovation to maximize the impact of clinical and translational research for child health across Emory and Children’s from bench-to-bedside.
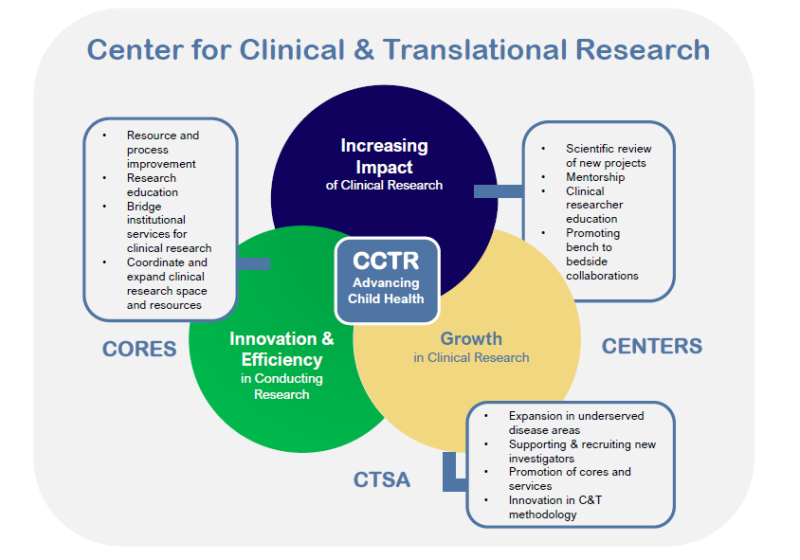
CCTR Services

Clinical and Translational Research Growth
CCTR bridges bench and clinical research to drive innovation between Emory and Children’s.

Research Operations
CCTR collaborates with Emory & Children's to streamline research administration operations.










