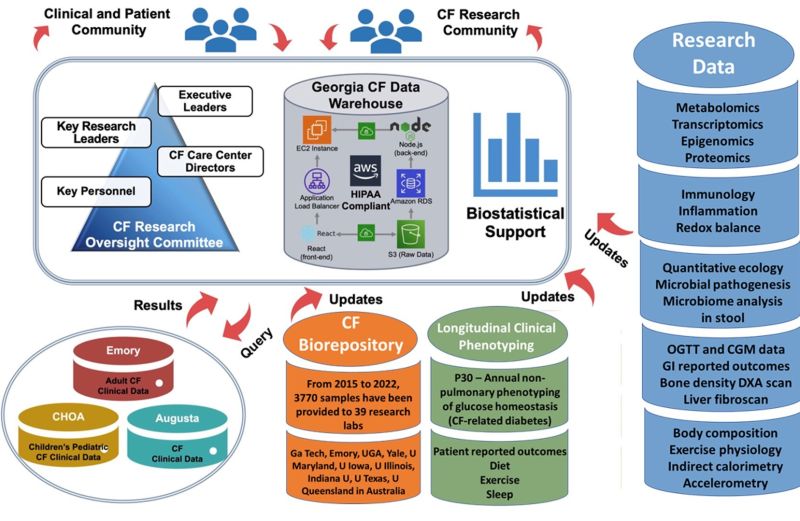
The complexity of CF-related disease requires advanced computational analysis to integrate diverse data. The CRI Core was established to accelerate CF research by uniting clinician scientists, biostatisticians, data scientists, and database engineers. The CRI core provides an infrastructure to ensure that human subject research oversight, sound experimental design principles, sophisticated data management, and innovative statistical and bioinformatics analyses are applied to each project from study design through data analysis and dissemination of new knowledge. Central to this is the Georgia CF Data Warehouse (GACFDW), which stores all CF-related data from our research network. The CRI Core enhances data validity, clarity, and interpretation, ensuring efficient and secure data accumulation and sharing.
The goal of the P30 GA CF Research and Translation Core Center is to promote interdisciplinary research into CF pathogenesis and translate new knowledge into therapies to improve the longevity and quality of life for people with CF. Our strategy focuses on the ~700 adult and pediatric CF patients in our clinical program, with the CRI Core facilitating this effort. The CRI Core aims to provide guidance and expertise in conducting CF human research, particularly on non-pulmonary aspects of CF disease. Arlene Stecenko, MD serves as core director, alongside co-directors Shasha Bai, PhD and Rishi Kamaleswaran, PhD. The CRI core includes the previously established CF Discovery Core.
CRI Core Services
Provide Translational and Clinical Research Support via the Research Oversight Committee (ROC)
The CRI Core provides training and oversight for designing and conducting CF human subject research at the Georgia CF Core Center. Its key service is the Research Oversight Committee (ROC), which guides the development of all CF studies to balance participant safety, research benefits, and efficiency for concurrent experimental protocols. Thus, the ROC functions as a regulatory, oversight, and advisory committee. Providing trainees and researchers access to clinical experts and research scientists at the very beginning of protocol development and grant submission will improve study design and enhance efficiency and scientific rigor.
Co-Chaired by Arlene Stecenko, MD, and Kymry Jones, PhD, the ROC comprises esteemed members including the Directors and Associate Directors of the DEG and NLB cores, P30 Biostatisticians Shasha Bai, PhD and Scott Gillespie, the CF Care Center Directors at Emory and Augusta, and the Technical Director of the CF Biospecimen Repository (CF-BR), Chris Driggers.
For more information about clinical samples provided by the CF-BR and associated clinical data, please visit the CF Discovery Core.
To inquire further about the ROC, please fill out the ROC Submission Form.
Provide Biostatistical and Bioinformatics Support
The CRI core provides statistical consultation for experimental design of clinical and translational research projects, power/sample size calculations, data analysis, and development of grant proposals and manuscripts. The CRI core also provides data management that provides a seamless interface between the type of specimens collected, the associated clinical data, and the biostatistical support team Shasha Bai, PhD and Scott Gillespie.
Provide Access to the Georgia CF Data Warehouse
The CRI Core provides a cloud-based computational infrastructure that supports access to integrated clinical and research data, enabling advanced bioinformatics analyses. The GA CF Data Warehouse (GACFDW) is a federated database system that combines and mines clinical and research data collected across our CF research network since 2015. This innovative system ensures CF researchers have a rich source of longitudinal and up-to-date information. Unlike traditional methods relying on ad hoc and offline data extraction, this real-time query system revolutionizes analysis processes.
By providing data access, sharing technical capabilities, automating data capture and retrieval, and ensuring data quality, this component of the CRI Core supports individual projects, encourages cross-collaboration among P30 cores, and attracts new investigators to the CF community.
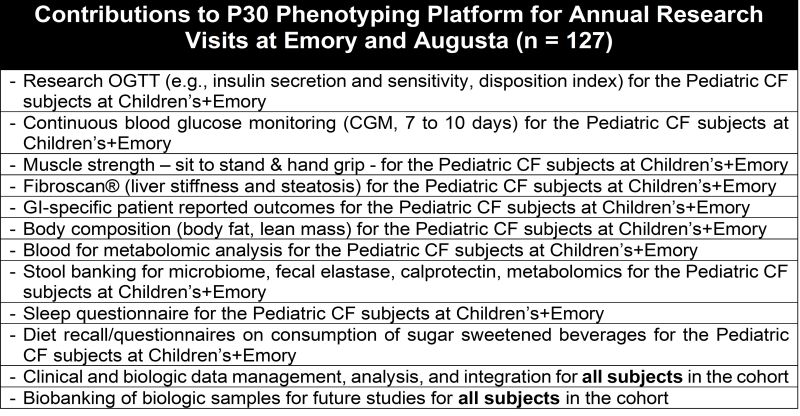
Central to this service is the integration of clinical and research data from a cohort of 127 CF subjects spanning toddlers to senior citizens, housed within the GACFDW. Accumulating since 2020 from the DEG and NLB cores, this data focuses on non-pulmonary CF manifestations. By Fall 2024, this initiative in comprehensive endotyping will be available to investigators, offering a crucial resource for CF research.